
Abstract (ESHRE 2023): Artificial intelligence (AI)-supported MAGENTA oocyte assessments shown to prospectively correlate with utilizable blastocyst development in patients, and for the first time in oocyte donors
STUDY QUESTION:
Can MAGENTA, an AI oocyte assessment tool, prospectively assess the quality of oocytes retrieved from patients and donors as it relates to reproductive outcomes?
SUMMARY ANSWER:
MAGENTA AI oocyte assessments provide insights on the quality of mature oocytes retrieved from both patients and young donors that correlate with utilizable blastocyst development.
WHAT IS KNOWN ALREADY:
Oocyte donation has increasingly become a treatment path for many patients struggling with infertility. With specific criteria, oocyte donation aims to provide higher quality oocytes to increase the chances of success for recipients. However, not all oocytes from even a young donor will be of the same quality or have the same chances of reproductive success. MAGENTA is a non-invasive oocyte AI image analysis tool that provides an assessment of oocyte quality through a score on a scale of 0-10. MAGENTA scores in patient oocytes have previously been shown to correlate with subsequent development of a blastocyst, and its quality.
STUDY DESIGN:
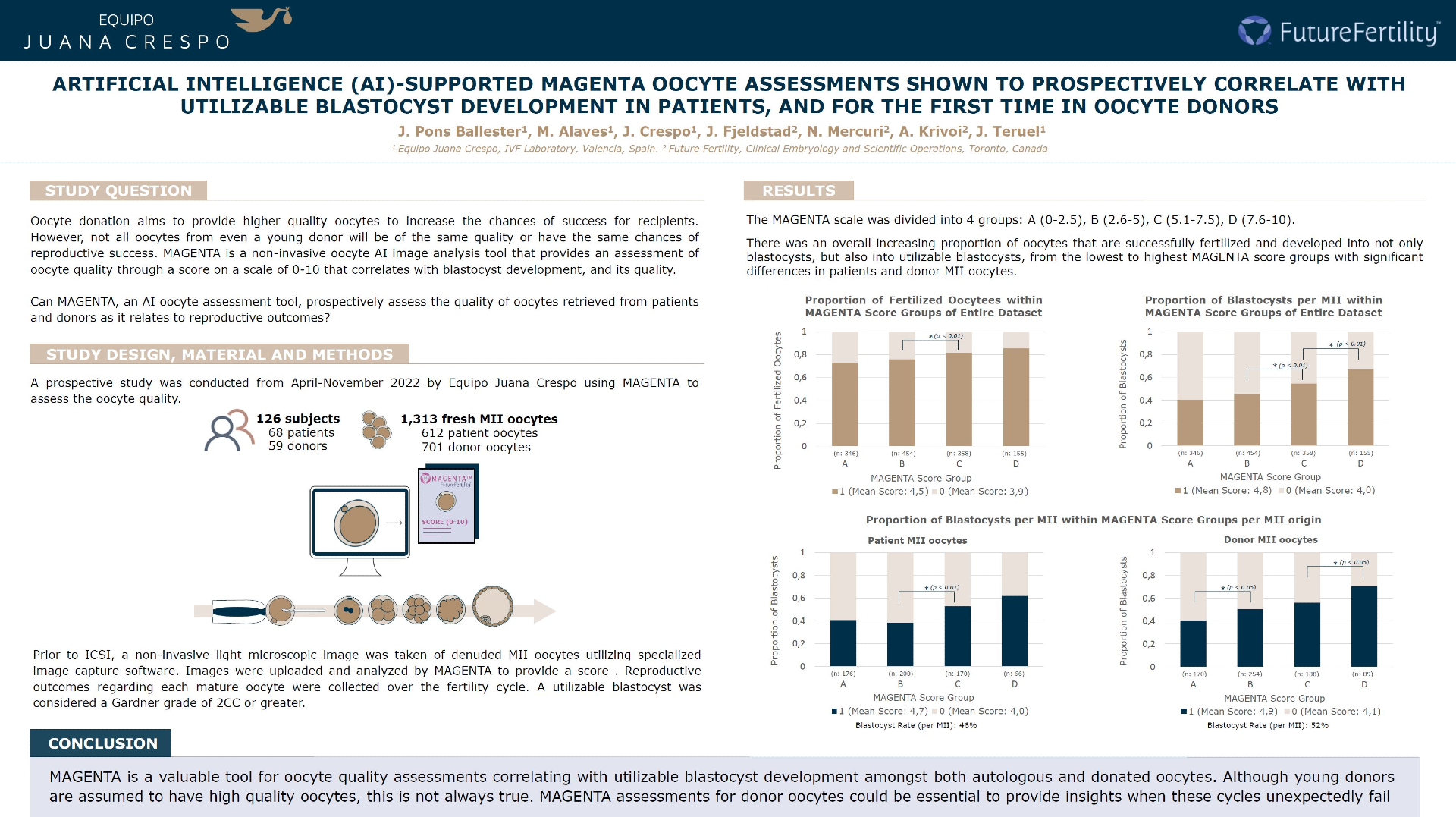
A prospective study was conducted from April-November 2022 by Equipo Médico Crespo (Valencia, Spain) utilizing MAGENTA to assess the oocyte quality of patient and donor oocytes by assigning a score on a scale of 0-10. MAGENTA scores of denuded metaphase II (MII) oocytes were assessed for correlation to reproductive outcomes (fertilization and blastocyst development). 1,313 fresh MII oocytes retrieved from 126 subjects (68 patients, 59 donors) between the ages of 19-47 were included for analysis.
METHODS:
Prior to ICSI, a non-invasive light microscopic image was taken of denuded MII oocytes utilizing specialized image capture software. Images were uploaded and analyzed by MAGENTA to provide a score, which remained blinded for the duration of the study. Reproductive outcomes regarding each mature oocyte were collected over the fertility cycle. Blastocyst development was defined by a Gardner grade by Day 5/6 post-ICSI. A utilizable blastocyst was considered a Gardner grade of 2CC or greater.
MAIN RESULTS AND THE ROLE OF CHANCE:
The following comparisons were assessed by Welch’s Two Sample t-tests.
Overall, successfully fertilized oocytes had higher mean MAGENTA scores (4.5) than those that did not (3.9) (p<0.01); furthermore, those oocytes that successfully developed into a blastocyst had higher mean MAGENTA scores (4.8) than those that did not (4.0) (p<0.01).
Patient oocytes were found significantly older (mean age: 38.5) compared to donated oocytes (mean age: 25) (p<0.01).
Patient oocytes that successfully developed into a blastocyst had a higher mean MAGENTA score (4.7) than those that did not (4.0) (p<0.01); similarly, donated oocytes that successfully developed into a blastocyst had a higher mean MAGENTA score (4.9) than those that did not (4.1) (p<0.01).
The MAGENTA scale was divided into 4 groups: A: 0-2.5 (346 oocytes); B: 2.6-5 (454 oocytes); C: 5.1-7.5 (358 oocytes); D: 7.6-10 (155 oocytes), over the whole dataset.
There was an overall increasing proportion of oocytes that developed into not only blastocysts, but more impressively, utilizable blastocysts from the lowest to highest MAGENTA score groups with significant differences between the patient oocytes scored in group B(38%) and C(50%) (p<0.05), and the donated oocytes scored in group A(39%) and B(52%), and group C(54%) and D(66%) (p<0.05) by Two Proportions Z-tests.
LIMITATIONS & REASONS FOR CAUTION:
This study was conducted in fresh oocyte ICSI cycles. The use of frozen patient and donor oocytes should be assessed by VIOLET prior to vitrification, which takes into consideration freezing and thawing statistics. Further data is required to determine any correlation of MAGENTA with embryo ploidy or implantation status.
WIDER IMPLICATIONS OF THE FINDINGS:
MAGENTA’s a valuable tool for oocyte quality assessments correlating with utilizable blastocyst development amongst both autologous and donated oocytes. Although young donors are assumed to have high quality oocytes, this is not always true. MAGENTA assessments for donor oocytes could be essential to provide insights when these cycles unexpectedly fail.
You Might Also Like …
Join our mailing list for dispatches on the future of fertility
